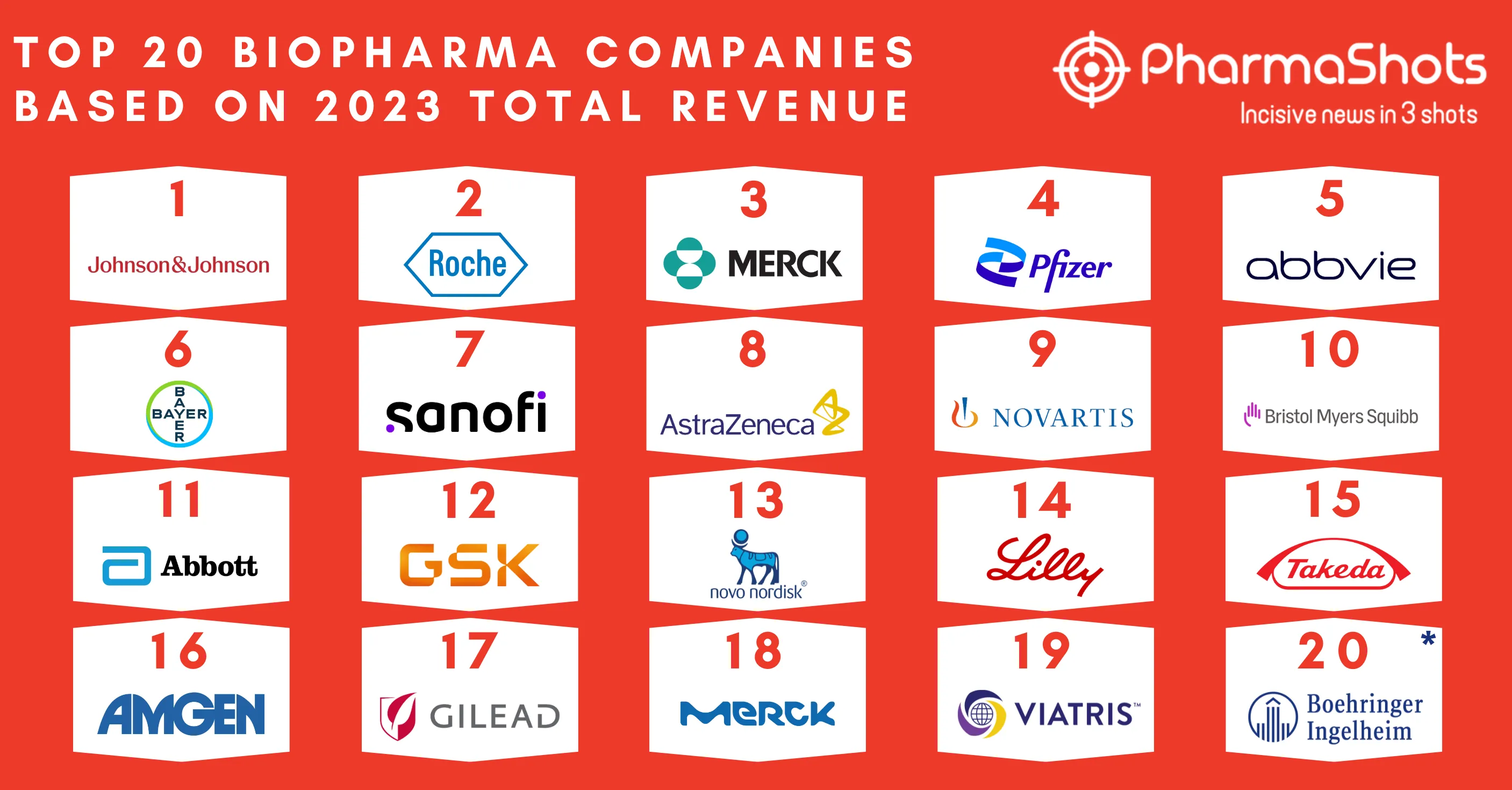
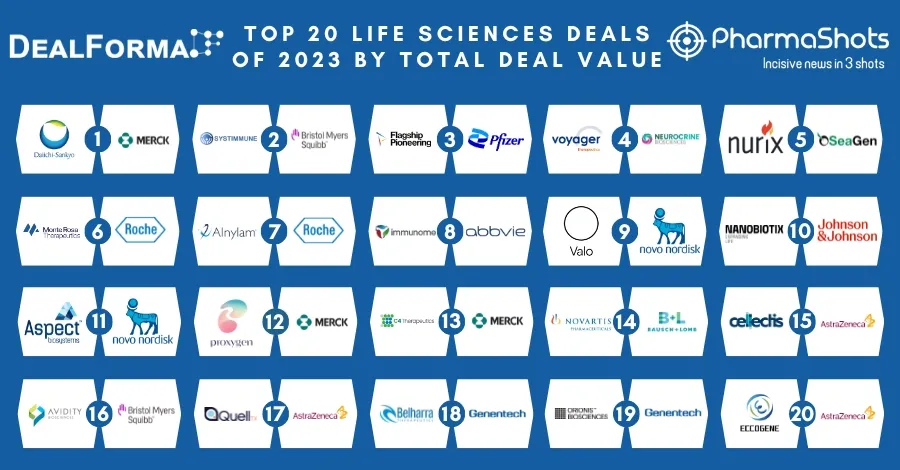
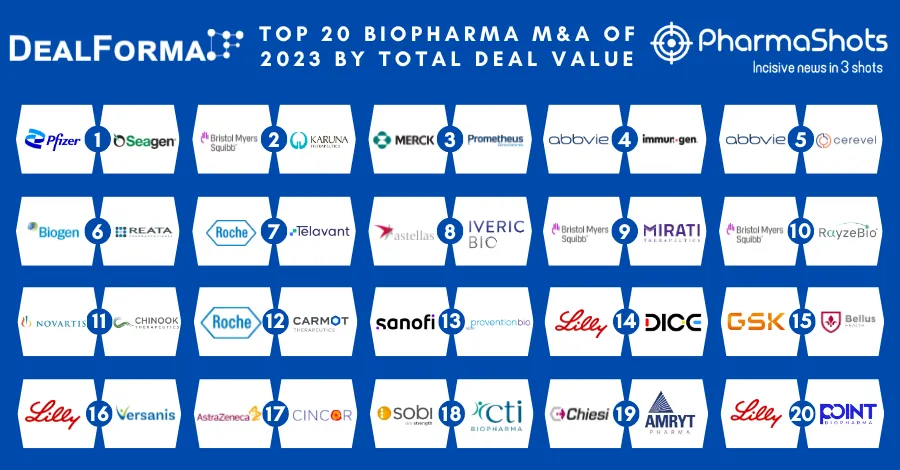
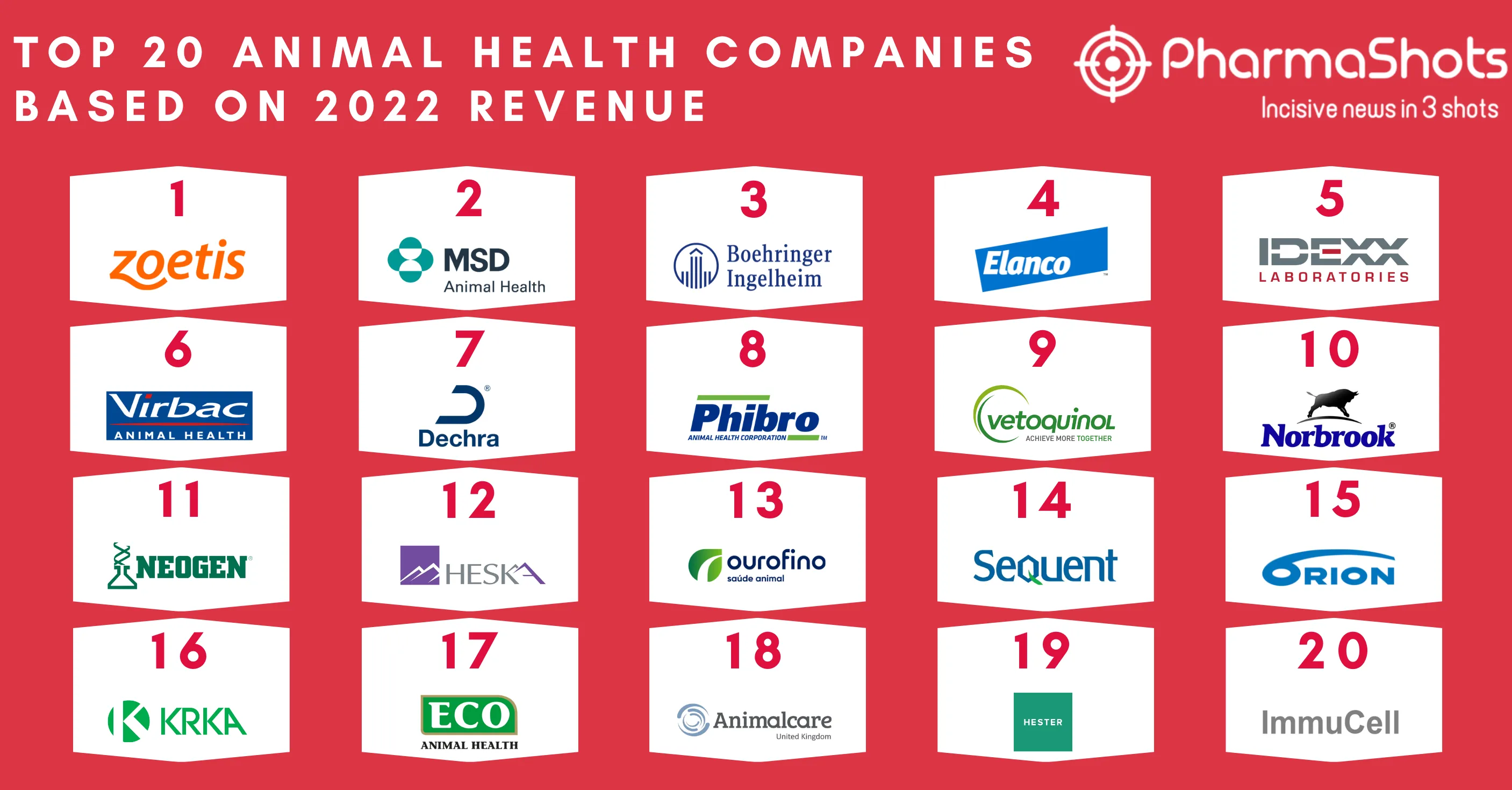
Merck and Moderna Initiate P-III (V940-001) Trial of V940 + Keytruda (pembrolizumab) for Adjuvant Treatment of Resected High-Risk (Stage IIB-IV) Melanoma
Shots:
- The companies initiate the P-III (V940-001) clinical trial evaluating the safety and efficacy of V940 (mRNA-4157) + Keytruda (anti-PD-1 therapy) vs Keytruda alone in a ratio (2:1) in 1089 patients with resected high-risk (Stage IIB-IV) melanoma at 165+ sites in ~25 countries globally
- The 1EPs of the study is RFS while the 2EPs incl. distant metastasis-free survival (DMFS), OS, and safety
- V940 (mRNA-based individualized neoantigen therapy) + Keytruda received the BTD & PRIME scheme from the US FDA and EMA for the adjuvant treatment of patients with high-risk melanoma, based on the results from the P-IIb study (KEYNOTE-942/mRNA-4157-P201) study
Ref: Merck | Image: Merck
PharmaShots! Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com
Click here to read the full press release
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.